Corvallis, Ore. – The vibrant blue discovered by Oregon State University researcher Mas Subramanian has cleared its final regulatory hurdle: The Environmental Protection Agency has approved its use for commercial purposes, including in paint for the artists who have long coveted it.
The approval was announced by the Shepherd Color Company, which licensed the pigment in 2015.
Through much of recorded human history, people around the world have sought inorganic compounds that could be used to paint things blue, often with limited success. Most had environmental or durability issues.
Known commercially as Blue10G513, the pigment discovered by Subramanian in 2009 is the first new inorganic blue in more than 200 years. It has rocked the color world since he and colleagues came upon it while experimenting with new materials for electronics applications.
Subramanian, distinguished professor of chemistry at OSU, and his team mixed manganese oxide – which is black in color – with other chemicals, then heated them in a furnace to nearly 2,000 degrees Fahrenheit.
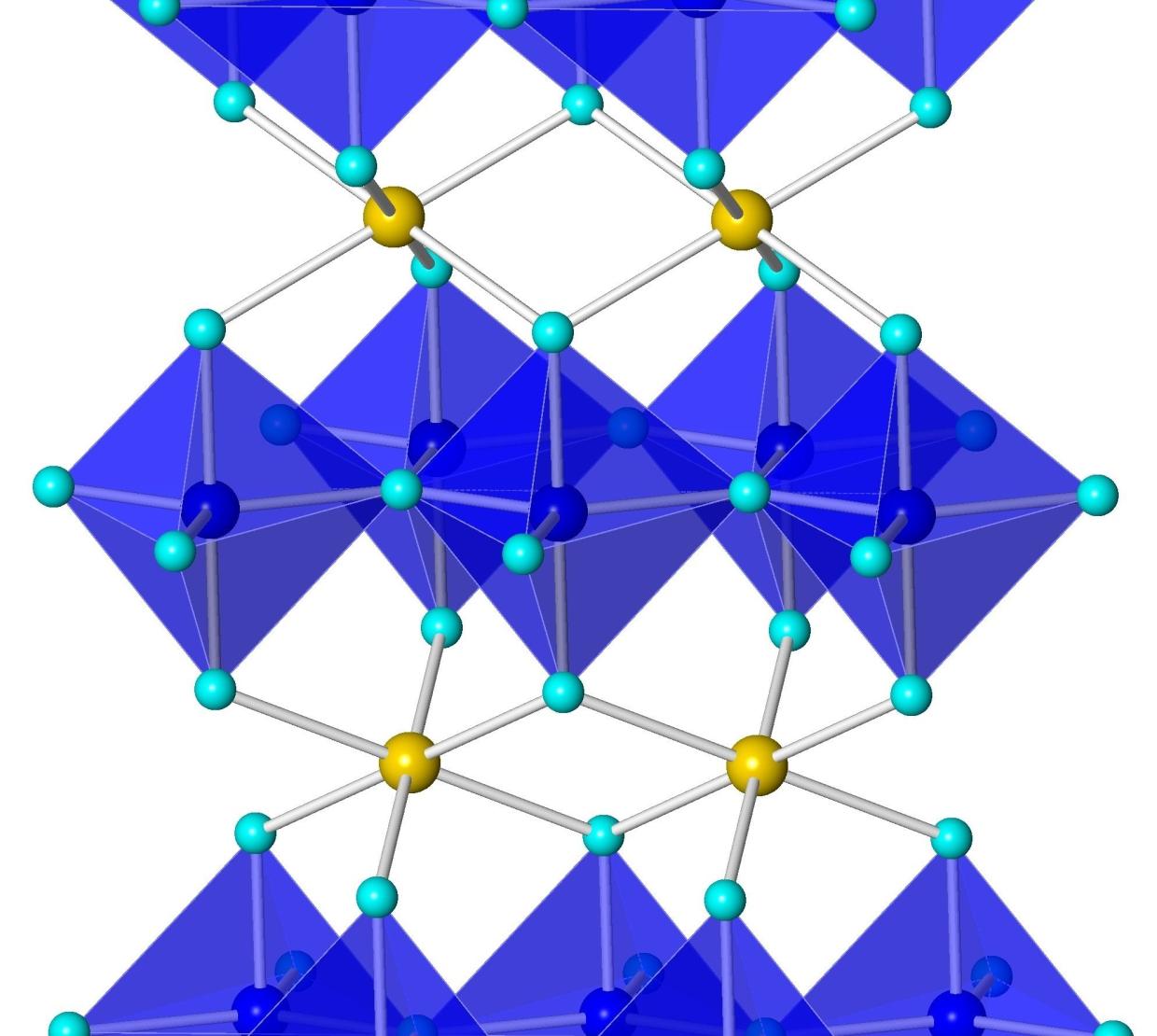
One of their samples turned out to be a brilliant blue, named YInMn blue after the component elements yttrium, indium and manganese. The pigment represented a huge advance in non-toxicity and stability as well as vividness.
YInMn blue features a unique structure that allows the manganese ions to absorb red and green wavelengths of light while reflecting only blue.
The Shepherd Color Company in 2017 received its first EPA approval for the pigment, a low-volume exemption that allowed it to be used in industrial coatings and plastics. Also in 2017, YInMn blue inspired a new Crayola crayon color: Bluetiful.
The pigment is so durable, and its compounds are so stable – even in oil and water – that the color does not fade. The pigment also reflects the infrared red part of sunlight, a characteristic that can help keep buildings painted with it cool.
Before YInMn blue, the last blue discovery was cobalt aluminum oxide-based blue, synthesized by a French chemist in 1802. Cobalt blue remains a dominant commercial pigment because of its intensity of color, ease of synthesis and wide applicability.
Its production, however, it requires a significant amount of a cobalt ion, Co2+, that’s hazardous to both humans and the environment.
“Most pigments are discovered by chance,” Subramanian said. “The reason is because the origin of the color of a material depends not only on the chemical composition, but also on the intricate arrangement of atoms in the crystal structure. So someone has to make the material first, then study its crystal structure thoroughly to explain the color.”
Subramanian, the Milton Harris Professor of Materials Science in the OSU College of Science, earlier this year received a special $200,000 grant from the National Science Foundation to pursue the holy grail of color research: an inorganic red pigment that’s vivid, safe and durable.
“We got lucky the first time with YInMn blue, and now we have come up with some design principles,” Subramanian said. “If successful, our research will discover bright, environmentally benign pigments that can be used by artists, in industrial paints and in other coating technologies. It would also bring a huge long-term payoff, not only as a commercial success but by advancing color chemistry and technology.”